Arsenic Reduction

The Crusader Arsenic Reduction Filter is designed to reduce Arsenic from microbiologically safe water at flow rates of up to 20 gallons per minute.
Why Reduce Arsenic in Water?
Arsenic is an abundant, naturally occurring element that can be found at varying concentrations in the Earth’s outer crust around the world. Inorganic arsenic compounds found in water are highly toxic while organic arsenic compounds (such as the arsenobetaine found in seafood) are generally less harmful to health.
It is important to understand that arsenic cannot be destroyed in the environment. It can only change its form. Most inorganic and organic arsenic compounds are white or colorless powders that do not evaporate, but can be attached to tiny particles that will inevitably become airborne.
When airborne particles are tiny enough, they can stay suspended by air currents for days and travel many thousands of miles from their original point of origin.
These particles will eventually hit the ground courtesy of gravity or precipitation (rain, snow etc…). Many common arsenic compounds can easily dissolve in water, so arsenic can get into lakes and rivers, and is not only found in groundwater.
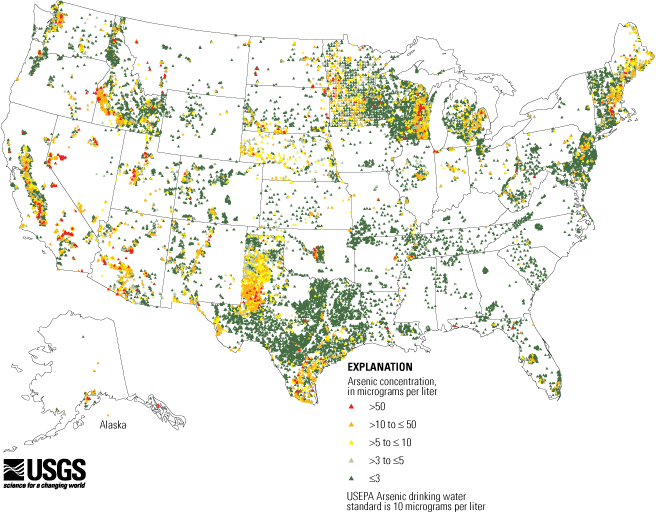
Currently, the primary recognized cause of unintentional arsenic consumption is from drinking groundwater that contains arsenic. Surveys of US drinking water indicate that about 80 percent of water supplies have less than 2 ppb of arsenic, but two percent of supplies exceed 20 ppb of arsenic.
Arsenic is an under-appreciated, growing threat.
Both inorganic and organic arsenic compounds are metabolized by the human body to varying degrees and some will be excreted in urine. Most organic arsenic is expelled and a large amount of inorganic will leave the body after a few weeks, but some of that arsenic will remain indefinitely.
An elevated level of arsenic is believed to interfere with cellular metabolism. Arsenic poisoning produces gastroenteritis, esophageal pain, vomiting and violent diarrhea. Eventually the skin becomes cold and clammy, blood pressure drops and overall body weakness sets in. Death from circulatory failure is sweet release from the convulsions. Large doses of arsenic insufficient to kill will cause restlessness, nausea, vomiting, headaches, dizziness, chills, cramps, irritability and variable levels of paralysis and neuropathy that may progress over several weeks.
Even at extremely low levels, arsenic consumption has recently been linked to the development of diabetes, oxidative stress, DNA damage, skin damage and immune system malfunction in susceptible persons.
The Department of Health and Human Services (DHHS), The International Agency for Research on Cancer (IARC) and US EPA have classified arsenic as a human carcinogen.
Chronic exposure to even low arsenic levels (less than 0.05 mg/L) has been linked to health complications, including cancer of the skin, kidney, lung and bladder, as well as other diseases of the skin, neurological and cardiovascular system. Skin absorption of arsenic is negligible; therefore, hand washing and bathing do not currently pose a known risk to human or animal health.
7 Common Types of Arsenic in Water:
| Name | Common Abbreviation | Chemical Formula | CAS # |
|---|---|---|---|
| Arsenous acid (arsenite) | AsIII | As(OH)3 | 13464-58-9 |
| Arsenic acid (arsenate) | AsV | AsO(OH)3 | 7778-39-4 |
| Monomethylarsonous acid | MMAIII | CH3As(OH)2 | 25400-23-1 |
| Monomethylarsonic acid | MMAV | CH3AsO(OH)2 | 124-58-3 |
| Dimethylarsinous acid | DMAIII | (CH3)2AsOH | 55094-22-9 |
| Dimethylarsinic acid | DMAV | (CH3)2AsO(OH) | 75-60-5 |
| Trimethylarsine oxide | TMAO | (CH3)3AsO | 4964-14-1 |
Water exposed to oxygen will generally contain arsenic in the pentavalent +5 (oxidized) state, whereas hypoxic waters (low or no dissolved oxygen) will contain arsenic in the trivalent +3 (reduced) state. Both are quite toxic; the trivalent is more easily assimilated by humans, making it even more dangerous.
Arsenic is one of the hardest ions to remove from water. It has a high molecular weight and there are many factors that will impact its removal from water. One of the main factors is that phosphate ions are very similar to arsenic ions, and compete for exchange sites. If the feed water has high levels of phosphate, the capacity for arsenic removal will be much lower.
Another factor that will affect the ability to remove arsenic from water is the feed pH. It is best to maintain close to neutral pH (~7 pH) for arsenic removal applications. At lower pH levels, arsenic can become insoluble and lose its ionic charge. The levels of natural arsenic in water will vary from area to area with the highest levels in areas with very deep wells.
Technical Specifications and Information:
Crusader Arsenic removal systems are engineered to work in single-tank or lead/lag configurations.
- NSF/ANSI Standard 61 Certified Media
- Proven iron chemistry
- No fines
- No backwash
- Centrally Regenerable Medial
- Optimal flow dynamics
- Rapid adsorption kinetics
- Low pressure drop
- Spent media passes Toxicity Characteristic Leaching Procedure (TCLP)
Factors impacting arsenic removal capacity
The capacity of Granular Iron Media (GIM) used for arsenic removal are significantly impacted by the following factors:
•pH – increasing pH results in lower capacity
•Phosphate – competes vigorously with arsenic for exchange sites on media
•Silica – competes for exchange sites and can precipitate and/or bind with other contaminants and block exchange sites
•Vanadium – competes vigorously with arsenic for exchange sites
•Other oxyanions (selenite, molybdate, antimonate, chromate) – will also have a negative effect on throughput capacity
•Specific flow rate – gpm/ft3 of media or BV/h – higher specific flow rates results in earlier breakthrough and lower capacity
•Empty Bed Contact Time (EBCT) – lower EBCT results in earlier breakthrough and lower capacity
This filtration system should only be installed after a comprehensive water test and engineering evaluation.
Essential Information required for designing a system
It is important to gather as much information as possible on the system so that “surprises” can be avoided. For example, arsenic III is typically non-ionic and will need to be oxidized prior to removal. Other anions and oxy-anions will compete with arsenic for removal by ion exchange resin so it is best to test for the following items prior to designing the treatment system:•Arsenic III (ppb)
•Arsenic V (ppb)
•Vanadium (measured to ppb levels, eg. 10 ppb )
•Phosphate (measured in ppb levels, eg. 30 ppb )
•Silica (ppm)
•pH
•Other oxyanions (e.g. molybdate, selenite, antimonate, uranyl—total all in ppb)
•Peak flowrate – gpm or lpm
•Water usage/day – GPD or LPD
•Target maximum arsenic level in treated water
Other factors that affect fouling, precipitation and oxidation, or that impact MCL limits:
•Suspended solids
•Total hardness
•Iron / manganese
•Chlorine or other oxidants
•Nitrate
•Microbiological count (if suspected to be a problem)
Special notes
The potential exists for nitrate dumping above the MCL due to residual anion capacity in the product. If nitrate in influent is above 5 ppm as N, contact your Crusader Technical Representative.
Copyrighted content reproduced with permission of the author